Electrochemical and theoretical insights into Schiff base derivatives for the prevention of copper corrosion
Abstract
This study reports the synthesis and characterization of a new Schiff base derivative, 2-(5-methyl-1H-pyrazol-3-yl)-N′-(2-(5-methyl-1H-pyrazol-3-yl)acetyl)acetohydrazide (AA3). The molecular structure of AA3 was confirmed using 1H and 13C nuclear magnetic resonance (NMR) spectroscopy. The corrosion inhibition performance of AA3 on copper in a 3.5 wt% NaCl solution was evaluated through electrochemical impedance spectroscopy (EIS), potentiodynamic polarization (PDP) techniques, and theoretical chemical studies, particularly density functional theory (DFT) calculations. Experimental data demonstrated that AA3 is an effective corrosion inhibitor, with its inhibition efficiency increasing in correlation with concentration. At a concentration of 5 × 10−4 M, AA3 achieved a notable inhibition efficiency of 79.66%. Scanning electron microscopy (SEM) analysis of copper surfaces treated with 10−3 M AA3 confirmed the formation of an adsorbed protective film, suggesting surface coverage. The adsorption characteristics of AA3 molecules aligned well with the Langmuir isotherm model. The 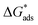 value of AA3 is −38.83 kJ mol−1, demonstrating that it acts on the metal/solution interface through physical adsorption and chemisorption. Theoretical calculations at the molecular scale illustrated that the nitrogen and oxygen atoms in the AA3 molecule play a key role in the inhibition process by interacting with copper atoms and blocking active sites. This was supported by the calculated quantum chemical descriptors. The highest occupied molecular orbital (EHOMO = −5.73 eV) and the lowest unoccupied molecular orbital (ELUMO = −0.17 eV) indicated a favorable electronic structure for adsorption and inhibition, similar to the energy gap (ΔE = 5.56 eV). These findings are further supported by Monte Carlo simulations, which revealed various probable interactions between the AA3 inhibitor and copper surface. The strong agreement between the theoretical predictions and experimental results provides significant insights into the mechanism of corrosion inhibition by AA3.
value of AA3 is −38.83 kJ mol−1, demonstrating that it acts on the metal/solution interface through physical adsorption and chemisorption. Theoretical calculations at the molecular scale illustrated that the nitrogen and oxygen atoms in the AA3 molecule play a key role in the inhibition process by interacting with copper atoms and blocking active sites. This was supported by the calculated quantum chemical descriptors. The highest occupied molecular orbital (EHOMO = −5.73 eV) and the lowest unoccupied molecular orbital (ELUMO = −0.17 eV) indicated a favorable electronic structure for adsorption and inhibition, similar to the energy gap (ΔE = 5.56 eV). These findings are further supported by Monte Carlo simulations, which revealed various probable interactions between the AA3 inhibitor and copper surface. The strong agreement between the theoretical predictions and experimental results provides significant insights into the mechanism of corrosion inhibition by AA3.



 Please wait while we load your content...
Please wait while we load your content...