Ring-size dependent ratiometric photoluminescence of cyclophane mechanophores†
Abstract
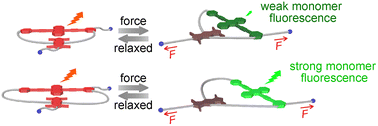
Mechanochromic mechanophores which instantly and reversibly change their photoluminescence color are suitable molecular probes for the in situ visualization and detection of forces that arise in polymers. Here, we report that the ring size significantly affects the ratiometric photoluminescence of cyclophane mechanophores whose working mechanism relies on the dissociation of intramolecular charge-transfer (CT) complexes. We compare three cyclophanes, which each feature a 9,10-bis(phenylethynyl)anthracene (BPA) group and one pyromellitic diimide (PMDI) in their cycle. These aromatic groups were connected with linkers of different lengths. In toluene solutions, all cyclophanes display suppressed BPA emission in comparison to a linear BPA model compound, and a red-shifted emission band associated with a BPA:PMDI CT complex is observed. The extent of CT formation scales inversely with the length of the spacers and is thus largest for the smallest cyclophane. The three cyclophanes were covalently incorporated into segmented polyurethane elastomers, which were processed into thin films. In the stress-free state, films of the three materials all display roughly equal monomer and CT emission intensity. Upon uniaxial deformation, this ratio changes in favor of the monomer emission. The changes were very pronounced for the polymer containing the largest cyclophane, and very small in the material containing the smallest mechanophore. Thus, the data show clearly that the mechanoresponse of cyclophane mechanophores strongly depends on the ring size, which dictates to what extent the chromophores can interact in the absence and presence of an applied force.


 Please wait while we load your content...
Please wait while we load your content...