Perceiving the influence of phenyl-carbazole isomers on sulfone/thioxanthone-based D–A–D hosts: realizing efficient red-phosphorescent OLEDs†
Abstract
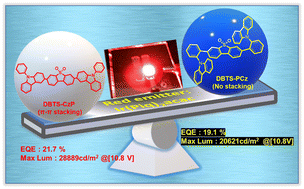
We successfully synthesized six host materials with a donor–acceptor–donor configuration. These hosts consist of donor units, 3-yl-9-phenyl-9H-carbazole (CzP) or 9-(o-phenyl)-9H-carbazole (PCz), connected to acceptor units including thioxanthone (TXO), diphenyl sulfone (DBTS), and spiro[fluorene-9,9′-thioxanthene] 10′,10′-dioxide (SpDBTS). The utilization of CzP or PCz donor units captured our interest as it influenced the molecular rigidity, electrochemical and photophysical properties, and their role as hosts for phosphorescent organic light-emitting diodes (PhOLEDs). The synthesized materials that possess triplet energy gaps higher than that of the red emitter (Ir(piq)2acac) exhibit efficient energy transfer capabilities, making them suitable candidates for use as hosts in the production of red PhOLEDs. We introduced an electron-transporting CN-T2T to construct a co-host emitting layer to attain charge balance. DBTS-CzP exhibited an outstanding photoluminescence quantum yield of 94% in pristine thin films. In a DBTS-CzP/CN-T2T co-host system, the device displayed outstanding electroluminescence properties, reaching a peak external quantum efficiency of 21.7%, a peak current efficiency of 13.4 cd A−1, and an impressively low turn-on voltage of 2.2 V. This finding suggests specific D–A–D molecular designs beneficial for realizing high-performance red phosphorescent OLEDs.


 Please wait while we load your content...
Please wait while we load your content...