Highly reversible lithium metal anodes enabled by a lithium sulfamate layer with high ionic conductivity and a low surface diffusion barrier†
Abstract
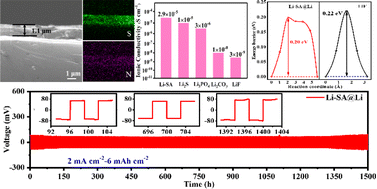
Lithium (Li) metal is considered as one of the most exciting anodes for next-generation batteries because of its high theoretical capacity and the lowest electrochemical redox potential. However, the long-standing issue of uncontrollable Li dendrite growth impedes the practical application of Li metal anodes in high-energy-density batteries. In this work, a promising principle of “simultaneous high ionic conductivity and low diffusion barrier” is developed to stabilize the Li metal anode. A lithium sulfamate (Li–SA) layer (∼1.1 μm) is in situ built on the Li metal surface through a facile one-step reaction between sulfamic acid and Li metal. The protective layer can effectively improve the moisture stability of Li metal and prevent the side reactions at the electrode/electrolyte interface. Moreover, the high ionic conductivity and the low surface diffusion barrier ensure the fast diffusion of Li ions through the thick artificial layer. As a result, the Li–SA coated Li anode (denoted as Li–SA@Li) enables a stable cycling at a large areal capacity of 6 mA h cm−2 at 2 mA cm−2 for 1500 h in Li||Li symmetric cells. When paired with the commercial LiFePO4 (LFP) or LiNi0.8Co0.1Mn0.1O2 (NCM) cathode, the Li–SA@Li anode demonstrates greatly enhanced rate performance and cycle stability compared with the bare Li counterpart. This finding will lay the foundation for designing a thick protective layer for high-rate and dendrite-free Li metal anodes.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators 2024


 Please wait while we load your content...
Please wait while we load your content...