Recent advances in energy-efficient chlorine production via HCl electrolysis
Abstract
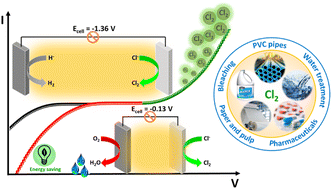
Chlorine (Cl2) is one of the most important and prime feeding reactants in the chemical industries, but around half of the chlorine employed in the industry gets converted into secondary products, mainly HCl. The conversion of HCl back to chlorine can be the best option for its maximum utilization. Although the catalytic conversion strategy is well established for the chemical recycling of chlorine, the drawbacks associated with it, such as the corrosion and stability of the catalyst, demand better alternatives. To overcome these issues, the electrolytic conversion of HCl for Cl2 and H2 generation is considered; however, high energy requirements and safety issues associated with this process call for its improvement. For this, the concept of replacing the cathodic hydrogen evolution reaction with the oxygen reduction reaction by introducing an oxygen depolarized cathode has been introduced as an energy-efficient and safe electrolysis approach. In this regard, this paper reviews the current trends in the efficient electrolysis of HCl, problems associated with it, and strategies employed to overcome it and puts forward its future perspectives.
- This article is part of the themed collection: Journal of Materials Chemistry A Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...