Potassium supporting electrolyte enhances stability of Ti-substituted polyoxovanadates for nonaqueous redox flow batteries†
Abstract
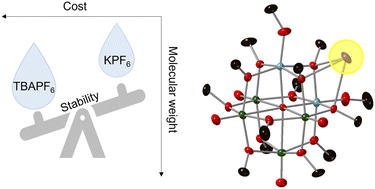
A bottleneck in the development of efficient and energy-dense electrochemical energy storage systems is limited strategies to enhance the stability of the charge carriers. While molecular engineering can allow desirable electrochemical properties of the active material to be achieved, understanding intermolecular interactions among species within the electrolyte can yield robust systems for practical applications. Here, we discuss the role of counter cations of the supporting electrolyte on the electrochemical stability and battery performance of the di-titanium substituted polyoxovanadate–alkoxide cluster, [Ti2V4O5(OMe)14]. Our results illustrate unique cation pairing effects associated with the use of alkali salts, MPF6 (M = Li+, K+), with the potassium-derived supporting electrolyte resulting in enhanced stability of reduced forms of the cluster. Single-crystal X-ray diffraction studies indicate that the cluster cores are linked by potassium atoms as contact ion-pairs. The results provide insight into the role of supporting electrolyte on the (electro)chemical stability of polyoxovanadate charge carriers.


 Please wait while we load your content...
Please wait while we load your content...
