Sequential proton coupled electron transfer events from a tetraruthenium polyoxometalate in photochemical water oxidation†
Abstract
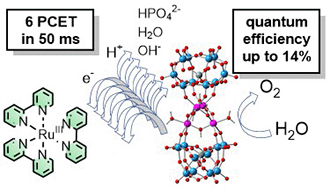
The tetraruthenium polyoxometalate {RuIV(H2O)4(μ-OH)2(μ-O)4[SiW10O36]2}10− (Ru4POM) shows multiple oxidative proton coupled electron transfer (PCET) events in a [Ru(bpy)3]2+/S2O82− photochemical cycle for catalytic water oxidation, with electrons conveyed to the photogenerated [Ru(bpy)3]3+ oxidant and protons transferred to aqueous bases. As shown by laser flash photolysis, in aqueous phosphate buffer the consumption of the [Ru(bpy)3]3+ oxidant by Ru4POM shows bi-exponential kinetics with a fast component and a slow component that feed the Ru4POM catalyst with up to 6 oxidative equivalents through PCET in ca. 50 ms. The apparent rates of both the fast and slow components depend linearly on HPO42− and on the pH of the aqueous medium, suggesting the involvement of the buffer base, of water and of OH− in assisting removal of the protons from Ru4POM. In particular, the beneficial role of HPO42− is reflected in a proportional improvement in the oxygen evolution activity, reaching quantum efficiency approaching 14%, although an excessive increase of buffer concentration is detrimental to the [Ru(bpy)3]3+ stability and leads to the abatement of the O2 evolution.
- This article is part of the themed collections: Recent Open Access Articles and Solar Fuels and Chemicals: Photocatalytic Water Splitting and CO2 Reduction


 Please wait while we load your content...
Please wait while we load your content...