Consecutive π-Lewis acidic metal-catalysed cyclisation/photochemical radical addition promoted by in situ generated 2-benzopyrylium as the photoredox catalyst†
Abstract
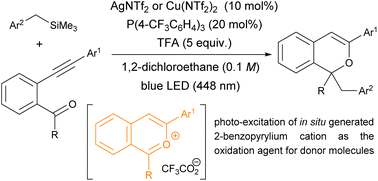
A π-Lewis acidic metal-catalysed cyclisation/photochemical radical addition sequence was developed, which utilises in situ generated 2-benzopyrylium cation intermediates as photoredox catalysts and electrophilic substrates to form 1H-isochromene derivatives in good yields in most cases. The key 2-benzopyrylium intermediates were generated through the activation of the alkyne moiety of ortho-carbonyl alkynylbenzene derivatives by such π-Lewis acidic metal catalysts as AgNTf2 and Cu(NTf2)2, and the subsequent intramolecular cyclisation and proto-demetalation using trifluoroacetic acid. Further photo-excitation of the 2-benzopyrylium intermediates facilitated single-electron transfer from a benzyltrimethylsilane derivative as a donor molecule to promote the radical addition of arylmethyl radicals to the 2-benzopyrylium intermediates.


 Please wait while we load your content...
Please wait while we load your content...