Excited-state antiaromaticity relief drives facile photoprotonation of carbons in aminobiphenyls†
Abstract
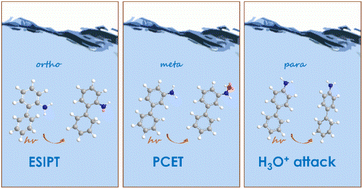
A combined computational and experimental study reveals that ortho-, meta- and para-aminobiphenyl isomers undergo distinctly different photochemical reactions involving proton transfer. Deuterium exchange experiments show that the ortho-isomer undergoes a facile photoprotonation at a carbon atom via excited-state intramolecular proton transfer (ESIPT). The meta-isomer undergoes water-assisted excited-state proton transfer (ESPT) and a photoredox reaction via proton-coupled electron transfer (PCET). The para-isomer undergoes a water-assisted ESPT reaction. All three reactions take place in the singlet excited-state, except for the photoredox process of the meta-isomer, which involves a triplet excited-state. Computations illustrate the important role of excited-state antiaromaticity relief in these photoreactions.


 Please wait while we load your content...
Please wait while we load your content...