A controlled non-radical chlorine activation pathway on hematite photoanodes for efficient oxidative chlorination reactions†
Abstract
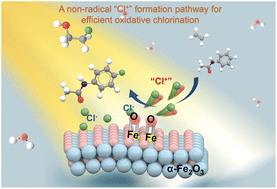
Photo(electro)catalytic chlorine oxidation has emerged as a useful method for chemical transformation and environmental remediation. However, the reaction selectivity usually remains low due to the high activity and non-selectivity characteristics of free chlorine radicals. In this study, we report a photoelectrochemical (PEC) strategy for achieving controlled non-radical chlorine activation on hematite (α-Fe2O3) photoanodes. High selectivity (up to 99%) and faradaic efficiency (up to 90%) are achieved for the chlorination of a wide range of aromatic compounds and alkenes by using NaCl as the chlorine source, which is distinct from conventional TiO2 photoanodes. A comprehensive PEC study verifies a non-radical “Cl+” formation pathway, which is facilitated by the accumulation of surface-trapped holes on α-Fe2O3 surfaces. The new understanding of the non-radical Cl− activation by semiconductor photoelectrochemistry is expected to provide guidance for conducting selective chlorine atom transfer reactions.


 Please wait while we load your content...
Please wait while we load your content...