Deciphering the doublet luminescence mechanism in neutral organic radicals: spin-exchange coupling, reversed-quartet mechanism, excited-state dynamics†
Abstract
Neutral organic radical molecules have recently attracted considerable attention as promising luminescent and quantum-information materials. However, the presence of a radical often shortens their excited-state lifetime and results in fluorescence quenching due to enhanced intersystem crossing (EISC). Recently, an experimental report introduced an efficient luminescent radical molecule, tris(2,4,6-trichlorophenyl)methyl-carbazole-anthracene (TTM-1Cz-An). In this study, we systematically performed quantum theoretical calculations combined with the path integral approach to quantitatively calculate the excited-state dynamics processes and spectral characteristics. Our theoretical findings suggest that the sing-doublet D1 state, originating from the anthracene excited singlet state, is quickly converted to the doublet 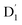 (trip-doublet) state via EISC, facilitated by a significant nonequivalence exchange interaction, with ΔJST = 0.174 cm−1. The formation of the quartet state (Q1, trip-quartet) was predominantly dependent on the exchange coupling 3/2JTR = 0.086 cm−1 between the triplet spin electrons of anthracene and the TTM-1Cz radical. Direct spin–orbit coupling ISC to the Q1 state was minimal due to the nearly identical spatial wavefunctions of the
(trip-doublet) state via EISC, facilitated by a significant nonequivalence exchange interaction, with ΔJST = 0.174 cm−1. The formation of the quartet state (Q1, trip-quartet) was predominantly dependent on the exchange coupling 3/2JTR = 0.086 cm−1 between the triplet spin electrons of anthracene and the TTM-1Cz radical. Direct spin–orbit coupling ISC to the Q1 state was minimal due to the nearly identical spatial wavefunctions of the 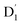 and Q1 levels. The effective occurrence of reverse intersystem crossing (RISC) from the Q1 to D1 state is a critical step in controlling the luminescence of TTM-1Cz-An. The calculated RISC rate kRISC, including the Herzberg–Teller effect, was 3.64 × 105 s−1 at 298 K, significantly exceeding the phosphorescence and nonradiative rates of the Q1 state, thus enabling the D1 repopulation. Subsequently, a strong electronic coupling of 37.4 meV was observed between the D1 and D2 states, along with a dense manifold of doublet states near the D1 state energy, resulting in a larger reverse internal conversion rate kRIC of 9.26 × 1010 s−1. Distributed to the D2 state, the obtained emission rate of kf = 2.98–3.18 × 107 s−1 was in quite good agreement with the experimental value of 1.28 × 107 s−1, and its temperature effect was not remarkable. Our study not only provides strong support for the experimental findings but also offers valuable insights for the molecular design of high-efficiency radical emitters.
and Q1 levels. The effective occurrence of reverse intersystem crossing (RISC) from the Q1 to D1 state is a critical step in controlling the luminescence of TTM-1Cz-An. The calculated RISC rate kRISC, including the Herzberg–Teller effect, was 3.64 × 105 s−1 at 298 K, significantly exceeding the phosphorescence and nonradiative rates of the Q1 state, thus enabling the D1 repopulation. Subsequently, a strong electronic coupling of 37.4 meV was observed between the D1 and D2 states, along with a dense manifold of doublet states near the D1 state energy, resulting in a larger reverse internal conversion rate kRIC of 9.26 × 1010 s−1. Distributed to the D2 state, the obtained emission rate of kf = 2.98–3.18 × 107 s−1 was in quite good agreement with the experimental value of 1.28 × 107 s−1, and its temperature effect was not remarkable. Our study not only provides strong support for the experimental findings but also offers valuable insights for the molecular design of high-efficiency radical emitters.



 Please wait while we load your content...
Please wait while we load your content...