Keto–enol equilibrium: stable tautomers of ortho-, meta-, and para-hydroquinones in large aromatics†
Abstract
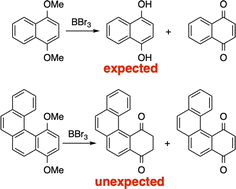
The synthesis of [6]helicene para-quinone starting from the 1,4-dimethoxy-[6]helicene derivative is presented. The demethylation reaction with boron tribromide led to unexpected results. Instead of the expected para-hydroquinone, the diketone tautomeric form was isolated. In contrast to the 1,4-hydroquinone and 1,4-dihydroxynaphthalene, the stable tautomers for the [4] and [6]helicenes were the aromatic diketones. These experimental results were corroborated by calculations. Additional calculations showed that these tautomeric species were indeed the stable forms of 1,4 and 1,3-hydroquinones when present in larger aromatics, in drastic contrast with 1,2-dihydroxy-aromatics (known as catechol).


 Please wait while we load your content...
Please wait while we load your content...