Stabilisation of solid-state cubic ammonia confined in a glass substance at ambient temperature under atmospheric pressure†
Abstract
Ammonia, a widely available compound, exhibits structural transitions from solid to liquid to gas depending on temperature, pressure, and chemical interactions with adjacent atoms, offering valuable insights into planetary science. It serves as a significant hydrogen storage medium in environmental science, mitigating carbon dioxide emissions from fossil fuels. However, its gaseous form, NH3(g), poses health risks, potentially leading to fatalities. The sublimation pressure (psub) of solid cubic ammonia, NH3(cr), below 195.5 K is minimal. In this study, we endeavoured to stabilise NH3(cr) at room temperature for the first time. Through confinement within a boric acid glass matrix, we successfully synthesised and stabilised cubic crystal NH3(cr) with a lattice constant of 0.5165 nm under atmospheric pressure. Thermodynamic simulations affirmed the stabilisation of NH3(cr), indicating its quasi-equilibrium state based on the estimated standard Gibbs energy of formation, 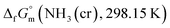 . Despite these advancements, the extraction of H2(g) from NH3(cr) within the boric acid glass matrix remains unresolved. The quest for an external matrix with catalytic capabilities to decompose inner NH3(cr) into H2(g) and N2(g) presents a promising avenue for future research. Achieving stability of the low-temperature phase at ambient conditions could significantly propel exploration in this field.
. Despite these advancements, the extraction of H2(g) from NH3(cr) within the boric acid glass matrix remains unresolved. The quest for an external matrix with catalytic capabilities to decompose inner NH3(cr) into H2(g) and N2(g) presents a promising avenue for future research. Achieving stability of the low-temperature phase at ambient conditions could significantly propel exploration in this field.

- This article is part of the themed collection: RSC Advances Physical Chemistry year in review 2024


 Please wait while we load your content...
Please wait while we load your content...