Hydrolytic dynamic kinetic resolution of racemic 3-phenyl-2-oxetanone to chiral tropic acid†
Abstract
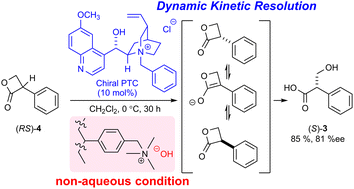
Tropic acid was synthesized in a good yield and with high enantioselectivity (81% ee) under non-biphasic conditions via the novel hydrolytic dynamic kinetic resolution of racemic 3-phenyl-2-oxetanone (tropic acid β-lactone) in the presence of a chiral quaternary ammonium phase-transfer catalyst and strongly basic anion exchange resin as the hydroxide ion donor.


 Please wait while we load your content...
Please wait while we load your content...