Cu(ii)-catalyzed aerobic oxidative coupling of furans with indoles enables expeditious synthesis of indolyl–furans with blue fluorescence†
Abstract
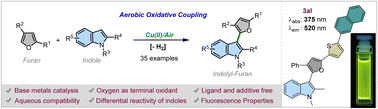
With the purpose of incorporating sustainability in chemical processes, there has been a renewed focus on utilizing earth-abundant metal catalysts to expand the repertoire of organic reactions and processes. In this work, we have explored the atom-economic oxidative coupling between two important electron-rich heterocycles – indoles and furans – using commonly available, inexpensive metal catalyst CuCl2·2H2O (<0.25$ per g) to develop an expeditious synthesis of indolyl–furans. Moreover, the reaction proceeded well in the presence of the so-called ‘ultimate oxidant’ – air, without the need for any external ligand or additive. The reaction was found to be scalable and to work even under partially aqueous conditions. This makes the methodology highly economical, practical, operationally simple and sustainable. In addition, the methodology provides direct access to novel indole–furan–thiophene (IFT)-based electron-rich π-conjugated systems, which show green-yellow fluorescence with large Stokes shift and high quantum yields. Mechanistic investigations reveal that the reaction proceeds through chemoselective oxidation of indole by the metal catalyst followed by the nucleophilic attack by furan.


 Please wait while we load your content...
Please wait while we load your content...