A comprehensive review of synthesis kinetics and formation mechanism of geopolymers
Abstract
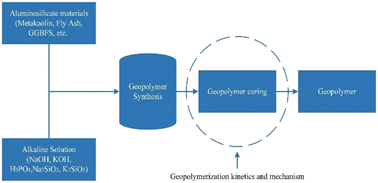
Geopolymers are synthesized by alkali or acid activation of aluminosilicate materials. This paper critically reviews the synthesis kinetics and formation mechanism of geopolymers. A variety of mechanistic tools such as Environmental Scanning Electron Microscopy (ESEM) and in situ Energy Dispersive X-ray diffractometry (EDXRD), in situ Isothermal Conduction Calorimetry (ICC), in situ Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR), 1H low-field Nuclear Magnetic Resonance (NMR) and Isothermal Conduction Calorimetry (ISC), and others and phenomenological models such as the John–Mehl–Avrami–Kolmogorov (JMAK) model, modified Jandar model, and exponential and Knudson linear dispersion models were used to study the geopolymerization kinetics and many mechanisms were proposed for the synthesis of geopolymers. The mechanistic tools and phenomenological models provided new insights about geopolymerization kinetics and formation mechanisms but each of the techniques used possesses some limitations. These limitations need to be removed and new methods or techniques must be developed to overcome these challenges and get more detailed information about all types of geopolymers. The formation mechanism consists of three to four stages such as dissolution of raw materials, polymerization of silica and alumina, condensation, and reorganization. The Si/Al ratio above the Si/Al ratio of reactants is more suitable and it increases the rate or degree of reaction and produces a higher compressive strength geopolymer. The Na/Al ratio of 1, water-to-solid (W/S) ratio of 0.30–0.45, a temperature in the range of 30 °C to 85 °C, and a curing time of 24 hours are the best for the synthesis of geopolymers. The growing demand for geopolymers in various fields requires the development of new advanced techniques for further understanding of kinetics and mechanisms for tailoring the properties of geopolymers for specific applications.
- This article is part of the themed collection: 2024 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...