Asymmetric copper-catalyzed alkynylallylic dimethylamination†
Abstract
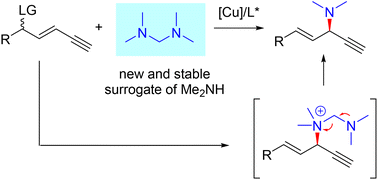
A feasible protocol for Cu-catalyzed asymmetric alkynylallylic dimethylamination is developed with the discovery of tetramethyldiaminomethane as a new, stable and convenient surrogate of dimethylamine. A series of enantioenriched 1,4-enynes are constructed in reasonable yields, high regioselectivities and moderate to good enantioselectivities. Mechanistic experiments show that the tertiary amine works as a nucleophile directly followed by the release of the expected dialkylamine unit, different from the conventional primary and secondary amines with the cleavage of a proton as the nucleophile. DFT calculations elucidate the origin of regio- and enantioselectivity for the present transformation.
- This article is part of the themed collection: Organic Chemistry Frontiers Emerging Investigator Series 2024–2025


 Please wait while we load your content...
Please wait while we load your content...