(o-CF3PhO)3P as a simple coupling reagent for direct amidation via activation of amines†
Abstract
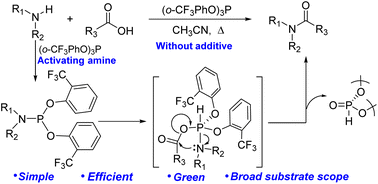
An unprecedented procedure for direct amidation is reported that employs a stoichiometric amount of the easy-to-access (o-CF3PhO)3P as a new and simple coupling agent. It is proposed that the developed reagent can be used for the activation of amines without any additives, unlike traditional coupling agents that activate carboxylic acids. This protocol readily allows the use of a variety of challenging substrates (including hindered and less reactive substrates or those containing sensitive functional groups) as well as the synthesis of Weinreb amides, lactams and conformationally maintained dipeptides, and its ability to enable the gram-scale synthesis of drugs and building blocks is demonstrated. Moreover, mechanistic insights into the activation of amines have been provided by a series of control experiments and DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...