Enantioselective synthesis of chiral BCPs
Abstract
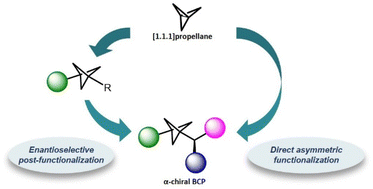
Bicyclo[1.1.1]pentanes (BCPs) have emerged as an interesting scaffold in drug design. These strained molecules can act as bioisosteres of para-substituted phenyl rings, tert-butyl groups or internal alkynes, leading to drug analogues with enhanced pharmacokinetic and physicochemical properties. Thus, catalytic methodologies for the synthesis of BCPs represent a major goal in modern organic synthesis. In particular, asymmetric transformations that provide chiral BCPs bearing an adjacent stereocenter are particularly valuable to expand the chemical space of this important scaffold. In this article, we discuss the available methodologies for the asymmetric synthesis of α-chiral BCPs, their key mechanistic features and their application in bioisosteric replacements in drug design.
- This article is part of the themed collection: 2024 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...