Reversible dual crosslinking in anthracenyl functionalized butyl elastomers based on ionic interaction and (4 + 4) cycloaddition mechanisms†
Abstract
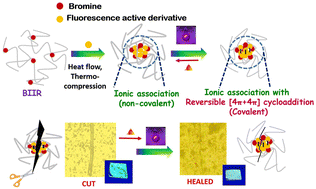
Elastomers are an important class of polymer materials used in commodities and industrial products. Irreversible crosslinked (sulphur or peroxide) networks improve their mechanical and thermal properties, but recycling, reprocessing, and remoulding are difficult. Designing reversible crosslinking in elastomers is extremely attractive for extending the product lifetime and recycling materials. Herein, a dual reversible crosslinking and fluorescence active elastomer is designed by synergistically introducing ionic interactions and [4 + 4] cycloaddition into bromo butyl rubber (BIIR). In this case, a fluorescence active 1,4-diazabicyclo[2.2.2]octane (DABCO)-anthracenyl derivative was synthesized and successfully functionalized onto the BIIR. The [4 + 4] cycloaddition of anthracenyl moieties contributed to dynamic covalent crosslinking, enhancing the mechanical strength of the BIIR ionomers. The resultant BIIR exhibits a tensile strength of 5.5 ± 0.3 MPa, significantly greater than that with ionic aggregation alone (2.5 ± 0.15 MPa). Because of dual responsive reversible networks, modified elastomers can undergo several reprocessing steps without considerable reduction in their mechanical performance, which differentiates the modified elastomers from sulfur crosslinked rubber. The present work provides a strategy for transforming commercial elastomers into self-healable and recyclable elastomers using ionic and cycloaddition approaches.


 Please wait while we load your content...
Please wait while we load your content...