Lewis acid-induced homo- and heterogeneous nickel catalysts for ethylene polymerization and copolymerization with polar monomers†
Abstract
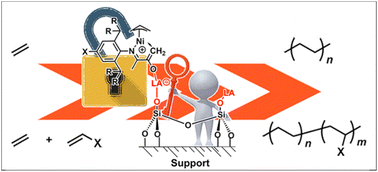
Lewis acids have been widely investigated to tune the properties of olefin polymerization catalysts. However, the application of this strategy in heterogeneous olefin–polar monomer copolymerization has rarely been studied. Herein, a series of [N, O]-type nickel catalysts bearing Lewis base response moieties was designed and synthesized. These catalysts can be modulated by Lewis acids such as B(C6F5)3 and MAO, resulting in greatly enhanced catalytic performances. This is due to the tuning of Lewis acid to the electronic and steric hindrance effects of the catalysts. Moreover, this Lewis acid–base combination was used as an anchoring strategy for heterogeneous catalysis, leading to increased thermal stability, the formation of ultra-high molecular weight polyethylene (Mn up to 205.3 × 104 g mol−1), and excellent morphology control. The immobilized nickel systems also promoted the copolymerization of ethylene with polar monomers, generating copolymer with high molecular weight and high activity.
- This article is part of the themed collection: Polymer Chemistry Emerging Investigators Series


 Please wait while we load your content...
Please wait while we load your content...