Stereoselective synthesis of gem-dihalopiperidines via the halo-aza-Prins cyclization reaction: access to piperidin-4-ones and pyridines†
Abstract
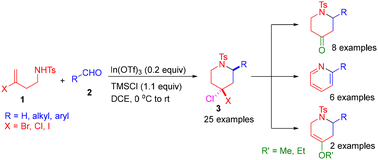
An efficient methodology for the synthesis of 4,4-dihalopiperidine derivatives in excellent yields has been developed using N-(3-halobut-3-en-1-yl)-4-methylbenzenesulfonamide and an aldehyde catalyzed by In(OTf)3. The reaction involves an initial formation of a six-membered carbocation via the aza-Prins cyclization reaction followed by a nucleophilic attack by a halide ion to give 4,4-dihalopiperidine. The dihalopiperidine is converted to tetrahydropiperidinone using Ac2O/Et3N in DCM/H2O (1 : 1). It is also utilized for the synthesis of pyridine scaffolds by treatment with DBU. Furthermore, the dihalopiperidine is transformed to its enol ether derivatives using KOH in alcohol.


 Please wait while we load your content...
Please wait while we load your content...