Non-enzymatic synthesis of C-methylated fluostatins: discovery and reaction mechanism†
Abstract
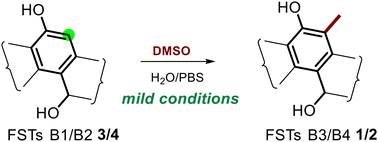
Two C-methylated fluostatins (FSTs) B3 (1) and B4 (2) were synthesized from flavin-mediated nonenzymatic epoxide ring-opening reactions of FST C. The structures of 1 and 2 were elucidated by HRESIMS, NMR, and ECD spectroscopic analyses. A subsequent 13C labeling study demonstrated that the C-methyl groups of 1 and 2 were derived from DMSO and enabled the mechanistic proposal of a nonenzymatic C-methylation.


 Please wait while we load your content...
Please wait while we load your content...