Phenol is a pH-activated linker to gold: a single molecule conductance study†
Abstract
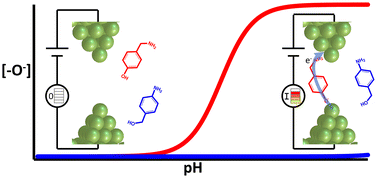
Single molecule conductance measurements typically rely on functional linker groups to anchor the molecule to the conductive electrodes through a donor–acceptor or covalent bond. While many linking moieties, such as thiols, amines, thiothers and phosphines have been used among others, very few involve oxygen binding directly to gold electrodes. Here, we report successful single molecule conductance measurements using hydroxy (OH)-containing phenol linkers and show that the molecule-gold attachment and electron transport are mediated by a direct O–Au bond. We find that deprotonation of the hydroxy moiety is necessary for metal–molecule binding to proceed, so that junction formation can be activated through pH control. Electronic structure and DFT+Σ transport calculations confirm our experimental findings that phenolate-terminated alkanes can anchor on the gold and show charge transport trends consistent with prior observations of alkane conductance with other linker groups. Critically, the deprotonated O−–Au binding shows features similar to the thiolate–Au bond, but without the junction disruption caused by intercalation of sulfur into electrode tips often observed with thiol-terminated molecules. By comparing the conductance and binding features of O–Au and S–Au bonds, this study provides insight into the aspects of Au-linker bonding that promote reproducible and robust single molecule junction measurements.


 Please wait while we load your content...
Please wait while we load your content...