Physicochemical surface properties of Chlorella vulgaris: a multiscale assessment, from electrokinetic and proton uptake descriptors to intermolecular adhesion forces†
Abstract
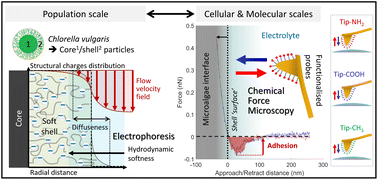
Given the growing scientific and industrial interests in green microalgae, a comprehensive understanding of the forces controlling the colloidal stability of these bioparticles and their interactions with surrounding aqueous microenvironment is required. Accordingly, we addressed here the electrostatic and hydrophobic surface properties of Chlorella vulgaris from the population down to the individual cell levels. We first investigated the organisation of the electrical double layer at microalgae surfaces on the basis of electrophoresis measurements. Interpretation of the results beyond zeta-potential framework underlined the need to account for both the hydrodynamic softness of the algae cells and the heterogeneity of their interface formed with the outer electrolyte solution. We further explored the nature of the structural charge carriers at microalgae interfaces through potentiometric proton titrations. Extraction of the electrostatic descriptors of interest from such data was obscured by cell physiology processes and dependence thereof on prevailing measurement conditions, which includes light, temperature and medium salinity. As an alternative, cell electrostatics was successfully evaluated at the cellular level upon mapping the molecular interactions at stake between (positively and negatively) charged atomic force microscopy tips and algal surface via chemical force microscopy. A thorough comparison between charge-dependent tip-to-algae surface adhesion and hydrophobicity level of microalgae surface evidenced that the contribution of electrostatics to the overall interaction pattern is largest, and that the electrostatic/hydrophobic balance can be largely modulated by pH. Overall, the combination of multiscale physicochemical approaches allowed a drawing of some of the key biosurface properties that govern microalgae cell–cell and cell–surface interactions.


 Please wait while we load your content...
Please wait while we load your content...