Design, synthesis, and anti-tumor activity of cyclic peptide–lenalidomide conjugated small molecules†
Abstract
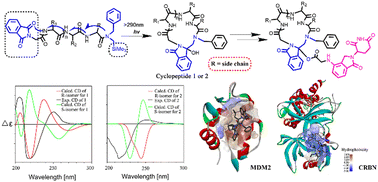
Cyclic peptides have emerged as significant contenders in drug development due to their exceptional specificity, enhanced metabolic stability, and superior cell permeability when compared to linear peptides. Many cyclic peptides have demonstrated potent anti-proliferative activity against a diverse range of malignant tumor cells. Lenalidomide has been extensively reported for its remarkable clinical efficacy in terms of anti-tumor, anti-inflammatory, and immunomodulatory effects. In this study, we successfully incorporated a lenalidomide fragment into designed cyclic peptide molecules to enhance their biological potency. In vitro studies clearly demonstrate that the antitumor activity of the conjugated molecules was superior to that of either a single cyclopeptide or lenalidomide. Preliminary cell staining experiments indicate that these conjugated molecules can induce autophagy and apoptosis. Furthermore, molecular docking studies were conducted to elucidate the binding patterns between the conjugated molecules and potential proteins. This research not only presents a novel approach for enhancing the biological activity of cyclic peptides but also opens up new avenues for exploring the synergy between small molecule drugs and peptides, thereby providing valuable insights into drug development.


 Please wait while we load your content...
Please wait while we load your content...