Unprecedented linear products by a mechanochemically activated Biginelli reaction using lawsone†
Abstract
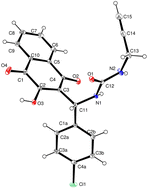
The Biginelli reaction, a crucial multicomponent reaction, was investigated involving 2-hydroxy-1,4-naphthoquinone (lawsone), p-substituted benzaldehydes, and ureas. Surprisingly, the classic Biginelli cyclized DHPM was not observed under various experimental conditions. Mechanochemical conditions, unlike traditional liquid phase conditions, led to the unprecedented formation of a series of ‘Biginelli-linear’ lawsone derivatives with high yields. The observed outcomes were consistent with DFT theoretical predictions, highlighting the preference for the Michael adduct under liquid conditions and the energetically implausible cyclization pathway for the classic DHPM compound. Additionally, the study achieved the novel cyclization of a ‘Biginelli-linear’ lawsone derivative into a cyclic carbamate for the first time.
- This article is part of the themed collection: RSC Mechanochemistry 2024 Hot Articles


 Please wait while we load your content...
Please wait while we load your content...