Discovery of aurones bearing two amine functionalities as SHIP2 inhibitors with insulin-sensitizing effect in rat myotubes†
Abstract
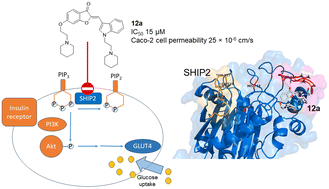
Pharmacological inhibition of the SH2 domain-containing inositol 5-phosphatase 2 (SHIP2) by small-molecule compounds presents an attractive approach to modulate insulin sensitivity. Few drug-like SHIP2 inhibitors have been discovered to date. A series of aurones incorporating key motifs from known SHIP2 inhibitors were synthesized and evaluated for SHIP2-inhibiting activity against a recombinant SHIP2 protein in vitro. Three aurones that inhibited SHIP2 at 15–50 μM were identified. These aurone inhibitors required two amine functionalities, one at ring A and a second at ring B for good inhibitory activity as exemplified by 12a. Mechanistically, molecular dynamics simulations revealed 12a to preferably bind to an allosteric site, restricting the motion of the flexible L4 loop required for SHIP2 phosphatase activity. Additionally, a basic piperidine moiety of 12a interacted with an aspartate residue proximal to the site. At 20–40 μM, 12a significantly enhanced glucose uptake in rat myotubes via increased Akt phosphorylation. 12a showed good permeability across the Caco-2 cell monolayer supporting the aurone chemotype as a new lead to develop drug-like, oral insulin sensitizers.


 Please wait while we load your content...
Please wait while we load your content...