Unlocking OER catalytic potential and chiral Fe3O4 film as a game-changer for electrochemical water oxidation pathway and by-product control†
Abstract
Electrochemical water splitting is an attractive technique for hydrogen production, but its development is constrained due to the sluggish kinetics of the oxygen evolution reaction (OER), limited charge transmission efficiency, and the generation of harmful by-products (like hydrogen peroxide) accompanying the OER process. Herein, we constructed an Fe3O4 film with an inherent chiral structure to demonstrate its chiral induced spin selectivity (CISS) effect on modulating the OER pathway and facilitating the transfer of electrons extracted from the OER. Compared with normal Fe3O4, the chiral Fe3O4 film facilitated the OER pathway for 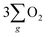 production via the CISS-induced spin alignment of electrons and radicals, while it suppressed the pathway for peroxide by-product generation. Meanwhile, as a half-metallic material, chiral Fe3O4 film promoted the transportation of spin-aligned electrons derived from water oxidation to an external circuit. Compared with normal Fe3O4 film which is not capable of aligning electrons, the potential at 10 mA cm−2 declined by 150 mV, the Tafel slope decreased by 104 mV dec−1, the charge transfer resistance decreased by 50%, the free charge carrier density was amplified by 10-fold, and the electron lifetime was prolonged within the chiral Fe3O4 film.
production via the CISS-induced spin alignment of electrons and radicals, while it suppressed the pathway for peroxide by-product generation. Meanwhile, as a half-metallic material, chiral Fe3O4 film promoted the transportation of spin-aligned electrons derived from water oxidation to an external circuit. Compared with normal Fe3O4 film which is not capable of aligning electrons, the potential at 10 mA cm−2 declined by 150 mV, the Tafel slope decreased by 104 mV dec−1, the charge transfer resistance decreased by 50%, the free charge carrier density was amplified by 10-fold, and the electron lifetime was prolonged within the chiral Fe3O4 film.



 Please wait while we load your content...
Please wait while we load your content...