Coupling of electrocatalytic CO2 reduction and CH4 oxidation for efficient methyl formate electrosynthesis†
Abstract
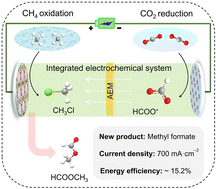
The electrocatalytic carbon dioxide reduction reaction (CO2RR) is promising for the conversion of greenhouse gases into value-added chemicals; however, it suffers from limited energy efficiency and product variability. The direct coupling of both cathode and anode products may provide new potential for carbon fixation and chemical electrosynthesis. Herein, we rationally designed a strategy to couple both CH4 oxidation reaction (CH4OR) and CO2RR in an integrated electrochemical system for the electrosynthesis of methyl formate. Using a bismuth (Bi) catalyst on the cathode and an IrO2 nanowire catalyst with Cl− on the anode, CO2 and CH4 gases were electrochemically reduced and oxidized into formate and CH3Cl, respectively, with industrial-level current densities and high selectivities. The formed HCOO− was transported under an electric field into the anode region and launched a nucleophilic attack on CH3Cl to produce methyl formate. This integrated electrochemical cell exhibited outstanding performance for coupling both CO2RR and CH4OR, including a peak methyl formate production rate of 1660 μmol h−1 cm−2, an energy efficiency of ∼15.2%, and excellent electrochemical stability at industrial-level current densities.


 Please wait while we load your content...
Please wait while we load your content...