Role of Fe decoration on the oxygen evolving state of Co3O4 nanocatalysts†
Abstract
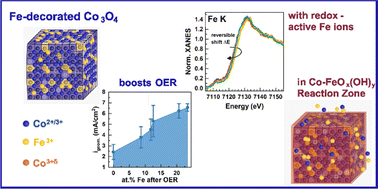
The production of green hydrogen through alkaline water electrolysis is the key technology for the future carbon-neutral industry. Nanocrystalline Co3O4 catalysts are highly promising electrocatalysts for the oxygen evolution reaction and their activity strongly benefits from Fe surface decoration. However, limited knowledge of decisive catalyst motifs at the atomic level during oxygen evolution prevents their knowledge-driven optimization. Here, we employ a variety of operando spectroscopic methods to unveil how Fe decoration increases the catalytic activity of Co3O4 nanocatalysts as well as steer the (near-surface) active state formation. Our study shows a link of the termination-dependent Fe decoration to the activity enhancement and a significantly stronger Co3O4 near-surface (structural) adaptation under the reaction conditions. The near-surface Fe– and Co–O species accumulate an oxidative charge and undergo a reversible bond contraction during the catalytic process. Moreover, our work demonstrates the importance of low coordination surface sites on the Co3O4 host to ensure an efficient Fe-induced activity enhancement, providing another puzzle piece to facilitate optimized catalyst design.
- This article is part of the themed collection: Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...