Assessing the importance of nitric acid and ammonia for particle growth in the polluted boundary layer†
Abstract
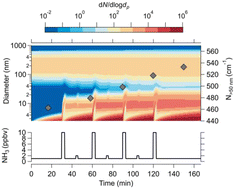
Aerosols formed and grown by gas-to-particle processes are a major contributor to smog and haze in megacities, despite the competition between growth and loss rates. Rapid growth rates from ammonium nitrate formation have the potential to sustain particle number in typical urban polluted conditions. This process requires supersaturation of gas-phase ammonia and nitric acid with respect to ammonium nitrate saturation ratios. Urban environments are inhomogeneous. In the troposphere, vertical mixing is fast, and aerosols may experience rapidly changing temperatures. In areas close to sources of pollution, gas-phase concentrations can also be highly variable. In this work we present results from nucleation experiments at −10 °C and 5 °C in the CLOUD chamber at CERN. We verify, using a kinetic model, how long supersaturation is likely to be sustained under urban conditions with temperature and concentration inhomogeneities, and the impact it may have on the particle size distribution. We show that rapid and strong temperature changes of 1 °C min−1 are needed to cause rapid growth of nanoparticles through ammonium nitrate formation. Furthermore, inhomogeneous emissions of ammonia in cities may also cause rapid growth of particles.


 Please wait while we load your content...
Please wait while we load your content...