Inducing ring distortions in unsubstituted metallophthalocyanines using axial N-heterocyclic carbenes†
Abstract
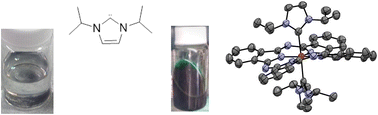
A series of metallophthalocyanine (PcM) complexes with axial N-heterocyclic carbene ligands (NHC; 1,3-diisopropylimidazol-2-ylidene (DIP) and 1,3-dimethylbenzimidazol-2-ylidene (DMB)) were prepared and structurally characterized. PcCoII(DIP), PcZnII(DIP), and PcZnII(DMB) are five-coordinate complexes with mild dome-type Pc-ring distortions, while PcFeII(DIP)2 is six-coordinate and has a very large ruffle-type ring-distortion with respect to typical PcM(L)2 systems. The distortion is induced by the highly steric axial DIP ligands. The distortions were quantified and classified by their bond lengths and torsion angles, and according to the normal-coordinate structural decomposition (NSD) analysis. Upon ligation of the NHC, the insoluble PcM materials were solublized in common organic solvents, with typical UV-visible Q-band maxima observable between 658 and 677 nm; the increased solubility is rationalized in terms of the reduced solid-state aggregation of the complexes, attributable to the axial ligation.
- This article is part of the themed collection: Articles behind our 2024 journal covers


 Please wait while we load your content...
Please wait while we load your content...