Theoretical study on hydroformylation catalyzed by cationic cobalt(ii) complexes†
Abstract
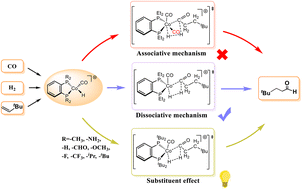
Hydroformylation is one of the most important homogeneous reactions in industrial production. Herein, a density functional theory (DFT) method was employed to investigate two proposed reaction mechanisms of hydroformylation catalyzed by cationic cobalt(II) complexes, the carbonyl dissociative mechanism and the associative mechanism. The calculated results showed that the heterolytic H2 activation is the rate-determining step for both the dissociative mechanism and the associative mechanism, with energy barriers of 26.8 kcal mol−1 and 40.5 kcal mol−1, respectively. Meanwhile, the regioselectivity, the spin multiplicity of the catalyst and the substituent effects on the reaction were also investigated. The most stable cobalt(II) catalyst has a doublet state and the linear aldehyde is the dominant product. In addition, it was found that the energy barrier of the reaction decreased when the electron density of the Co center of the catalyst was increased by changing the ligand. The catalytic activity of the catalyst was proposed to be the best when the PEt2 group of the ligand is replaced by the P(tBu)2 group. This study might not only provide new insights for hydroformylation catalyzed by cobalt but also facilitate theory-guided design of novel transition metal catalysts for hydroformylation.


 Please wait while we load your content...
Please wait while we load your content...