Exploring the electronic and steric effects on the dimerization of intramolecular frustrated Lewis pairs: a comparison between aminoboranes and aminoalanes†
Abstract
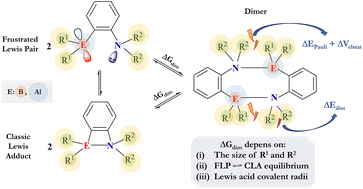
The dimerization of intramolecular aminoborane and aminoalane frustrated Lewis pairs was investigated using density functional theory. We systematically varied the substituents to gradually increase their bulkiness, including H, CH3, t-Bu, Ph, and Mes groups. Starting from the most stable conformer of the monomers, a frustrated Lewis pair or classic Lewis adduct, we studied the dimerization process for all systems, revealing significant variations in the Gibbs free energy. Dimerization was favored in four aminoboranes and six aminoalanes, depending on the specific combinations of substituents. Applying an energy decomposition analysis, we found that the preparation energy of the monomers and the non-orbital interactions between them are the primary contributors to the observed energetic differences, showing a clear linear relationship. Additionally, we analyzed the electronic effects by increasing the acidity of the Lewis acid, observing a shift toward endergonic and exergonic directions in aminoboranes and aminoalanes, respectively. This shift was attributed to the stabilization of a classic Lewis adduct. This study underscores three crucial factors influencing dimer formation: (i) substituent size, (ii) stabilization of the classic Lewis adduct conformation, and (iii) covalent radii of the Lewis centers. Understanding these factors is essential for designing FLPs and preventing unwanted dimerization that could affect their catalytic performance in H2 activation processes.


 Please wait while we load your content...
Please wait while we load your content...