Analyses of the electronic structures of FeFe-cofactors compared with those of FeMo- and FeV-cofactors and their P-clusters†
Abstract
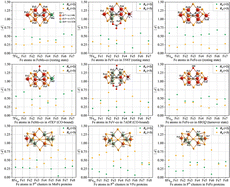
The electronic structures of FeFe-cofactors (FeFe-cos) in resting and turnover states, together with their PN clusters from iron-only nitrogenases, have been calculated using the bond valence method, and their crystallographic data were reported recently and deposited in the Protein Data Bank (PDB codes: 8BOQ and 8OIE). The calculated results have also been compared with those of their homologous Mo- and V-nitrogenases. For FeFe-cos in the resting state, Fe1/2/4/5/6/7/8 atoms are prone to Fe3+, while the Fe3 atom shows different degrees of mixed valences. The results support that the Fe8 atom at the terminal positions of FeFe-cos possesses the same oxidation states as the Mo3+/V3+ atoms of FeMo-/FeV-cos. In the turnover state, the overall oxidation state of FeFe-co is slightly reduced than those in the resting species, and its electronic configuration is rearranged after the substitution of S2B with OH, compatible with those found in CO-bound FeV-co. Moreover, the calculations give the formal oxidation states of 6Fe2+–2Fe3+ for the electronic structures of PN clusters in Fe-nitrogenases. By the comparison of Mo-, V- and Fe-nitrogenases, the overall oxidation levels of 7Fe atoms (Fe1–Fe7) for both FeFe- and FeMo-cos in resting states are found to be higher than that of FeV-co. For the PN clusters in MoFe-, VFe- and FeFe-proteins, they all exhibit a strong reductive character.
- This article is part of the themed collection: Articles behind our 2024 journal covers


 Please wait while we load your content...
Please wait while we load your content...