A mesoionic carbene stabilized nickel(ii) hydroxide complex: a facile precursor for C–H activation chemistry†
Abstract
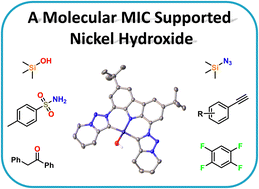
We report the synthesis of a new nickel(II) hydroxide complex 2 supported by a rigid, tridentate triazolylidene-carbazolid ligand. The complex can be accessed in high yields following a simple and stepwise extraction protocol using dichloromethane and aqueous ammonium chloride followed by aqeous sodium hydroxide solution. We found that complex 2 is highly basic, undergoing various deprotonation/desilylation reactions with E–H and C–H acidic and silylated compounds. In this context we synthesized a variety of novel, functionalized nickel(II) complexes with trimethylsilylolate (3), trityl sulfide (4), tosyl amide (5), azido (6), pyridine (7), acetylide (8, 9), fluoroarene (10 & 11) and enolate (12) ligands. We furthermore found that 2 reacts with malonic acid dimethyl ester in a knoevennagel-type condensation reaction, giving access to a new enolate ligand in complex 13, consisting of two malonic acid units. Furthermore, complex 2 reacts with acetonitrile to form the cyanido complex 14. The formation of complexes 13 and 14 is particularly interesting, as they underline the potential of complex 2 in both C–C bond formation and cleavage reactions.
- This article is part of the themed collection: Celebrating International Women’s Day 2025: Women in Inorganic Chemistry


 Please wait while we load your content...
Please wait while we load your content...