Mechanistic study of a coke-resistance Ni/ZrO2 catalyst for dry reforming of methane under external electric fields: a combined first-principles and microkinetic modeling study†
Abstract
The dry reforming of methane (DRM) reaction is environmentally friendly and economically efficient as it converts greenhouse gases to syngas. However, the lower ability of CO2 activation in the DRM reaction compared to methane cracking results in carbon deposition and eventual deactivation of Ni-based catalysts. Reducible oxide supports and electric fields can modify the electronic structure of Ni catalysts to improve the activity and anti-carbon deposition performance. Herein, the DRM reaction on a Ni16/ZrO2 catalyst under electric fields was comprehensively investigated using tetragonal phase ZrO2 with excellent activity under reaction conditions as a support loaded with Ni16 nanorods and combining density functional theory calculations with microkinetic modeling. The results showed that a strong interaction exists between Ni16 and ZrO2, with ZrO2 obtaining extra electrons from the Ni16 cluster. Compared with Ni(111), the ZrO2 support enhanced the adsorption strength of species on Ni catalysts, and the DRM reactivity exhibited the same trend. Electric fields can increase the activity of DRM reactions because positive electric fields promote methane activation and CHx oxidation, whereas negative electric fields are beneficial for CO2 activation. The Brønsted–Evans–Polanyi (BEP) linear trend still holds true even in the presence of external electric fields. The optimal DRM reaction path on Ni16/ZrO2 under different electric fields is CH–O. Based on microkinetic results, CH–CH* is the major mechanism for coke formation over Ni16/ZrO2. The DRM reactivity of Ni-based catalysts under positive electric fields was higher, along with more carbon deposition. Conversely, favorable CO2 activation with negative electric fields reduced carbon accumulation. The degree of rate control analysis showed that the activation of methane and CO2 and the oxidation of 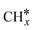 species are essential for the DRM reaction activity. This work revealed the effect of electric fields on the catalytic activity and deactivation mechanism of Ni-supported catalysts in DRM reactions.
species are essential for the DRM reaction activity. This work revealed the effect of electric fields on the catalytic activity and deactivation mechanism of Ni-supported catalysts in DRM reactions.



 Please wait while we load your content...
Please wait while we load your content...