Stable mass-selected AuTiOx nanoparticles for CO oxidation†
Abstract
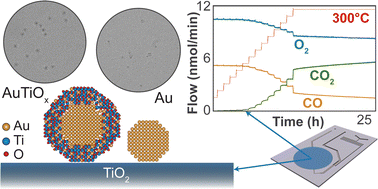
Stability under reactive conditions poses a common challenge for cluster- and nanoparticle-based catalysts. Since the catalytic properties of <5 nm gold nanoparticles were first uncovered, optimizing their stability at elevated temperatures for CO oxidation has been a central theme. Here we report direct observations of improved stability of AuTiOx alloy nanoparticles for CO oxidation compared with pure Au nanoparticles on TiO2. The nanoparticles were synthesized using a magnetron sputtering, gas-phase aggregation cluster source, size-selected using a lateral time-of-flight mass filter and deposited onto TiO2-coated micro-reactors for thermocatalytic activity measurements of CO oxidation. The AuTiOx nanoparticles exhibited improved stability at elevated temperatures, which is attributed to a self-anchoring interaction with the TiO2 substrate. The structure of the AuTiOx nanoparticles was also investigated in detail using ion scattering spectroscopy, X-ray photoelectron spectroscopy, and transmission electron microscopy. The measurements showed that the alloyed nanoparticles exhibited a core–shell structure with an Au core surrounded by an AuTiOx shell. The structure of these alloy nanoparticles appeared stable even at temperatures up to 320 °C under reactive conditions, for more than 140 hours. The work presented confirms the possibility of tuning catalytic activity and stability via nanoparticle alloying and self-anchoring on TiO2 substrates, and highlights the importance of complementary characterization techniques to investigate and optimize nanoparticle catalyst designs of this nature.
- This article is part of the themed collections: 2024 PCCP HOT Articles and Size effects in chemistry & physics of atomic & molecular clusters, nanoparticles & nanostructures


 Please wait while we load your content...
Please wait while we load your content...