Non-monotonic Soret coefficients of aqueous LiCl solutions with varying concentrations†
Abstract
We investigate the thermodiffusive properties of aqueous solutions of lithium chloride, using thermal diffusion forced Rayleigh scattering in a concentration range of 0.5–2 mole per kg of solvent and a temperature range of 5 to 45 °C. All solutions exhibit non-monotonic variations of the Soret coefficient ST with a concentration exhibiting a minimum at about one mole per kg of solvent. The depth of the minimum decreases with increasing temperature and shifts slightly towards higher concentrations. We compare the experimental data with published data and apply a recent model based on overlapping hydration shells. Additionally, we calculate the ratio of the phenomenological Onsager coefficients 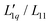 using our experimental results and published data to calculate the thermodynamic factor. Simple linear, quadratic and exponential functions can be used to describe this ratio accurately, and together with the thermodynamic factors, the experimental Soret coefficients can be reproduced. The main conclusion from this analysis is that the minimum of the Soret coefficients results from a maximum in the thermodynamic factor, which appears itself at concentrations far below the experimental concentrations. Only after multiplication by the (negative) monotonous Onsager ratio does the minimum move into the experimental concentration window.
using our experimental results and published data to calculate the thermodynamic factor. Simple linear, quadratic and exponential functions can be used to describe this ratio accurately, and together with the thermodynamic factors, the experimental Soret coefficients can be reproduced. The main conclusion from this analysis is that the minimum of the Soret coefficients results from a maximum in the thermodynamic factor, which appears itself at concentrations far below the experimental concentrations. Only after multiplication by the (negative) monotonous Onsager ratio does the minimum move into the experimental concentration window.

- This article is part of the themed collection: Celebrating International Women’s day 2025: Women in physical chemistry


 Please wait while we load your content...
Please wait while we load your content...