Dioxygen–halogen bonding exemplified by crystalline peroxosolvates of N,N′-bis(haloacetyl) bispidines†
Abstract
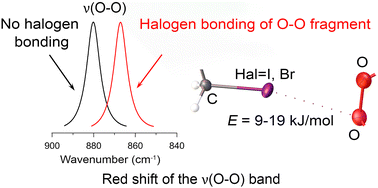
The halogen bonding in molecular crystals and supramolecular assemblies has been widely investigated. Special attention is given to the molecular structures capable of simultaneously exhibiting different types of non-covalent interactions, including conventional hydrogen bonds and halogen bonds. This paper systematically analyzes crystalline peroxosolvates of bispidine-based bis-amide derivatives, containing haloacetic acid residues, namely previously reported 1,1′-(1,5-dimethyl-3,7-diazabicyclo[3.3.1]nonane-3,7-diyl)bis(2-iodooethanone) peroxosolvate C13H20I2N2O2·H2O2 (1) and four new crystalline compounds, 1,1′-(1,5-dimethyl-3,7-diazabicyclo[3.3.1]nonane-3,7-diyl)bis(2-bromoethanone) peroxosolvate C13H20Br2N2O2·H2O2 (2), 1,1′-(9-hydroperoxy-9-hydroxy-1,5-dimethyl-3,7-diazabicyclo[3.3.1]nonane-3,7-diyl)bis(2-iodoethanone) peroxosolvate C13H20I2N2O5·0.5H2O2 (3), 1,1′-(9-hydroperoxy-9-hydroxy-1,5-dimethyl-3,7-diazabicyclo[3.3.1]nonane-3,7-diyl)bis(2-bromoethanone) peroxosolvate C13H20Br2N2O5·H2O2 (4), and 1,1′-(9-hydroperoxy-9-hydroxy-1,5-dimethyl-3,7-diazabicyclo[3.3.1]nonane-3,7-diyl)bis(2-chloroethanone) peroxosolvate C13H20Cl2N2O5·H2O2 (5). Compounds 2–5 were synthesized for the first time and their crystal structures were determined by single-crystal X-ray diffractometry (SCXRD). To the best of our knowledge, 3–5 are unprecedented crystalline hydrogen peroxide adducts of organic hydroperoxides (R-OOH). Short intermolecular contacts between halogen and hydroperoxo oxygen atoms were found in 1–3. The halogen bonding of C–I(Br) fragments with dioxygen species in compounds 1–3 as well as in the previously reported cocrystal of diacetone diperoxide with triodotrinitrobenzene (6) was identified through reduced density gradient analysis, Hirshfeld surface analysis, and Bader analysis of crystalline electron density. The interactions were quantified using the electron density topological properties acquired from the periodic DFT calculations and evaluated to lie in the range of 9–19 kJ mol−1. A distinctive spectral feature was revealed for this type of interaction, involving a red shift of the characteristic O–O stretching vibration by about 6 cm−1, which appeared in IR spectra as a narrow low-intensity band in the region 837–872 cm−1.


 Please wait while we load your content...
Please wait while we load your content...