Modeling Henry's law and phase separations of water–NaCl–organic mixtures with solvation and ion-pairing
Abstract
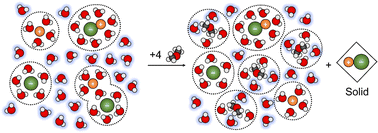
Empirical measurements of solution vapor pressure of ternary acetonitrile (MeCN) H2O–NaCl–MeCN mixtures were recorded, with NaCl concentrations ranging from zero to the saturation limit, and MeCN concentrations ranging from zero to an absolute mole fraction of 0.64. After accounting for speciation, the variability of the Henry's law coefficient at vapor–liquid equilibrium (VLE) of MeCN ternary mixtures decreased from 107% to 5.1%. Solute speciation was modeled using a mass action solution model that incorporates solute solvation and ion-pairing phenomena. Two empirically determined equilibrium constants corresponding to solute dissociation and ion pairing were utilized for each solute. When speciation effects were considered, the solid–liquid equilibrium of H2O–NaCl–MeCN mixtures appear to be governed by a simple saturation equilibrium constant that is consistent with the binary H2O–NaCl saturation coefficient. Further, our results indicate that the precipitation of NaCl in the MeCN ternary mixtures was not governed by changes in the dielectric constant. Our model indicates that the compositions of the salt-induced liquid–liquid equilibrium (LLE) boundary of the H2O–NaCl–MeCN mixture correspond to the binary plateau activity of MeCN, a range of concentrations over which the activity remains largely invariant in the binary water–MeCN system. Broader comparisons with other ternary miscible organic solvent (MOS) mixtures suggest that salt-induced liquid–liquid equilibrium exists if: (1) the solution displays a positive deviation from the ideal limits governed by Raoult's law; and (2) the minimum of the mixing free energy profile for the binary water-MOS system is organic-rich. This work is one of the first applications of speciation-based solution models to a ternary system, and the first that includes an organic solute.
- This article is part of the themed collection: 2023 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...