Structural analysis of anti-retroviral drug raltegravir and its potential impurity C: investigation of solubility and stability†
Abstract
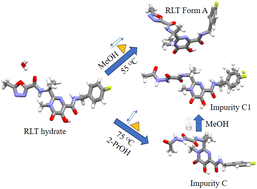
Raltegravir (RLT) is a human immunodeficiency virus integrase strand transfer inhibitor that is prescribed for the treatment of HIV-1 infection. The drug belongs to biopharmaceutics classification system (BCS) class II category and has poor aqueous solubility and chemical stability in acidic/basic medium. Polymorph screening was performed to search for an alternative solid form with improved drug attributes. By serendipity, this screening gave anhydrous RLT (form A) and two crystalline solid phases of impurity C. The RLT form A and impurity C were characterised using powder X-ray diffraction (XRD), thermal analysis (differential scanning calorimetry/thermogravimetric analysis) and vibrational spectroscopy (Fourier-transform infrared). The crystal structures of form A and impurity C were determined from the high resolution powder XRD patterns using Rietveld refinement, thus revealing their conformational and synthon differences. The crystal structure of impurity C1 (MeOH solvate) was solved from single crystal XRD and demonstrates the packing and conformational differences compared to parent phase C. The dissolution/solubility experiments confirmed that form A has a higher solubility than its hydrate in aqueous ethanol medium. Compared to the commercialized RLT potassium salt (RLT-K), form A did not offer improved chemical stability during acid hydrolysis (high-performance liquid chromatography data). Rather, impurity C exhibited higher thermal stability and aqueous stability compared to other RLT solid forms in an acidic medium.


 Please wait while we load your content...
Please wait while we load your content...