Synthesis, structure and stereodynamics of atropisomeric N-chloroamides†
Abstract
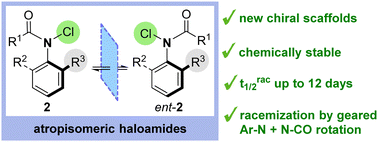
Atropisomeric N-chloroamides were efficiently accessed by electrophilic halogenation of ortho-substituted secondary anilides. The stereodynamics of atropisomerism in these novel scaffolds was interrogated by detailed experimental and computational studies, revealing that racemization is correlated with amide isomerization. The stereoelectronic nature of the amide was shown to significantly influence racemization rates, with potentially important implications for other C–N atropisomeric scaffolds.


 Please wait while we load your content...
Please wait while we load your content...