Aluminyl derived ethene functionalization with heteroallenes, leading to an intramolecular ligand rearrangement†
Abstract
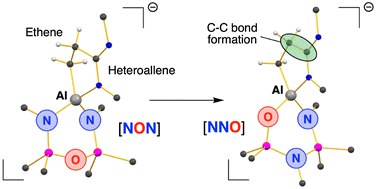
The aluminacyclopropane K[Al(NON)(η-C2H4)] ([NON]2− = [O(SiMe2NDipp)2]2−, Dipp = 2,6-iPr2C6H3) reacts with CO2 and iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr to afford ring-expanded products of C–C bond formation. The latter system undergoes a 1,3-silyl retro-Brook rearrangement of the NON-group, to afford the [NNO]2− ligand ([NNO]2− = [N(Dipp)SiMe2N(Dipp)SiMe2O]2−). The mechanism of transformation was examined by density functional theory (DFT).
NiPr to afford ring-expanded products of C–C bond formation. The latter system undergoes a 1,3-silyl retro-Brook rearrangement of the NON-group, to afford the [NNO]2− ligand ([NNO]2− = [N(Dipp)SiMe2N(Dipp)SiMe2O]2−). The mechanism of transformation was examined by density functional theory (DFT).


 Please wait while we load your content...
Please wait while we load your content...