Hydroxy-directed peptide bond formation from α-amino acid-derived inert esters enabled by boronic acid catalysis†
Abstract
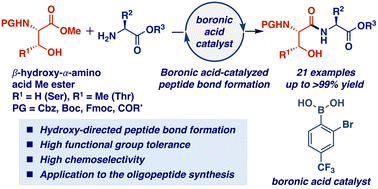
A boronic acid-catalyzed peptide bond formation from α-amino acid methyl esters is described. The catalysis showed high chemoselectivity for β-hydroxy-α-amino esters, affording the peptides in high to excellent yields with high functional group tolerance. This hydroxy-directed peptide bond formation could be applicable to oligopeptide syntheses. This is the first successful example of organoboron-catalyzed peptide bond formation from α-amino acid-derived inert esters.


 Please wait while we load your content...
Please wait while we load your content...