Ruthenium catalyzed transformation of levulinic acid to γ-valerolactone in water†
Abstract
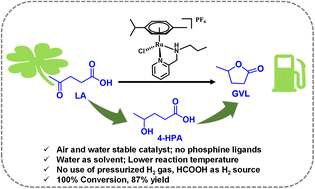
High catalytic activity for selective and efficient transformation of levulinic acid (LA) to γ-valerolactone (GVL) in water was achieved over (η6-p-cymene)Ru(II)-pyridylamine, [(η6-C10H14)RuCl(κ2-L)]+ (L = Namine-substituted pyridylamine ligands) based molecular catalysts. A series of complexes with pyridylamine ligands having different electronic and steric properties were synthesized and characterized. A significant influence of the Namine-substituents of the pyridylamine ligand on the catalytic activity was observed where the [(η6-p-cymene)RuCl(κ2-pyNHnpr)]+ catalyst ([Ru]-2) outperformed others with 87% yield and >99% selectivity for GVL at 80 °C in water. Advantageously, the activity of [Ru]-2 was also scaled up to gram scale transformation of LA to GVL. Control experiments, pH dependent NMR and mass studies revealed the involvement of crucial reaction intermediates and catalytic species in the transformation of LA to GVL.


 Please wait while we load your content...
Please wait while we load your content...