Direct conversion of carboxylic acids to free thiols via radical relay acridine photocatalysis enabled by N–O bond cleavage†
Abstract
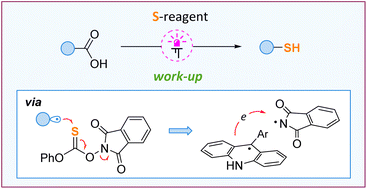
Carboxylic acids and thiols are basic chemical compounds with diverse utility and widespread reactivity. However, the direct conversion of unprotected acids to thiols is hampered due to a fundamental problem – free thiols are incompatible with the alkyl radicals formed on decarboxylation of carboxylic acids. Herein, we describe a concept for the direct photocatalytic thiolation of unprotected acids allowing unprotected thiols and their derivatives to be obtained. The method is based on the application of a thionocarbonate reagent featuring the N–O bond. The reagent serves both for the rapid trapping of alkyl radicals and for the facile regeneration of the acridine-type photocatalyst.


 Please wait while we load your content...
Please wait while we load your content...