Mechanistic studies on the formation of ternary oxides by thermal oxidation of the cubic laves phase CaAl2†
Abstract
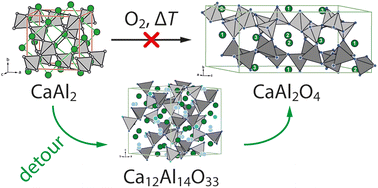
Oxide materials are of key importance in many aspects of everyday life. However, their solid-state syntheses require high temperatures and often multiple steps when conducted from the binary oxides. Herein, we report a proof-of-concept investigation addressing the possibility to synthesize oxides from a phase-pure, well-defined, and highly crystalline intermetallic starting material via oxidation with elemental oxygen. The thermal oxidation behavior of the cubic Laves phase CaAl2 was investigated under various atmospheric environments by thermal analysis or by different bulk synthesis techniques. Besides different furnace types, also varying O2 concentrations and different heating rates, and annealing times were explored. Interestingly, the reaction progresses via the intermediate Ca12Al14O33 (12 CaO·7 Al2O3) before the expected stoichiometric oxidation product, monoclinic CaAl2O4 (CaO·Al2O3), is finally observed. This is highly surprising, since the intermediate has a different Ca to Al ratio compared to the starting material. Different strategies were employed to optimize the synthetic conditions and to decipher the reaction mechanism. The formation of the various products was followed by a detailed analysis of the powder X-ray diffraction data via Rietveld refinements and additionally by 27Al MAS NMR experiments, while quantum-chemical calculations supported the proposed reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...