Lewis acid-catalyzed one-pot thioalkenylation of donor–acceptor cyclopropanes using in situ generated dithiocarbamates and propiolates†
Abstract
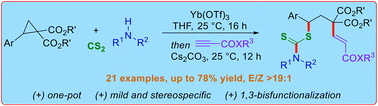
Lewis acid-catalyzed one-pot 1,3-thioalkenylation of donor–acceptor (D–A) cyclopropanes has been demonstrated employing in situ generated dithiocarbamates (from amines and CS2) as nucleophilic triggers and alkyl propiolates as electrophiles. This method addresses the limitations of previously known carbothiolation approach, eliminating the need for extra filtration prior to the subsequent trapping with electrophiles. The anticipated thioalkenylated products were obtained in good to excellent yields with a moderate to good E/Z ratio. Three new bonds (C–N, C–S, and C–C) are formed during this 1,3-bisfunctionalization reaction. Notably, employing enantiomerically pure D–A cyclopropanes resulted in enantiopure 1,3-thioalkenylated products, underscoring the stereospecific nature of the developed reaction.
- This article is part of the themed collection: Celebrating the 100th birthday of Professor Sukh Dev


 Please wait while we load your content...
Please wait while we load your content...